Welcome to Pharmacy India, your trusted source for top-tier pharmaceutical education resources! 🌟 Unlock a deeper understanding of Social Pharmacy with our comprehensive collection of Multiple Choice Questions (MCQs) tailored for the D. Pharma Exit Exam 1st Year. Dive into a wealth of insights, meticulously designed to enhance your exam preparation and ensure success in this dynamic field.
As a networked organization linking many centers of excellence, THSTI is envisioned as a collective of scientists, engineers and physicians that will effectively enhance the quality of human life through integrating a culture of shared excellence in research, education and…
What is H3N2 Influenza? A subtype of influenza called H3N2 In India, a virus has been rapidly spreading, resulting in 90 cases and 2 fatalities thus far. According to reports, the ailment causes a fever that lasts three to five days…
Biocon Biologics Chennai RND team is looking for enthusiastic research professional skilled in different aspects of cell culture process development in the development of biologics/biosimilars. Seeking candidates with experience in process development, quality-modulation, scale-up, tech-transfer, and/or process characterization. Qualification: M….
As part of the Pharmacovigilance Plan of India, the National Medical Commission (NMC) has ordered all accredited medical colleges and independent PG institutions to enrol as Adverse Drug Reaction Monitoring Centers (AMCs) with the Indian Pharmacopoeia Commission (IPC) as soon…
A vulnerable population in India is now being helped to survive abrupt cardiac arrests thanks to MedLern HeartCode full training. The MedLern platform is made exclusively for hospitals in India to standardise and enhance training for their medical staff. It…
The first patient with hepatocellular carcinoma (HCC) has begun treatment in phase 2a with the candidate drug fostroxacitabine bralpamide (fostrox) in combination with Lenvima, according to Medivir AB, a pharmaceutical company dedicated to creating novel cancer treatments in regions with…
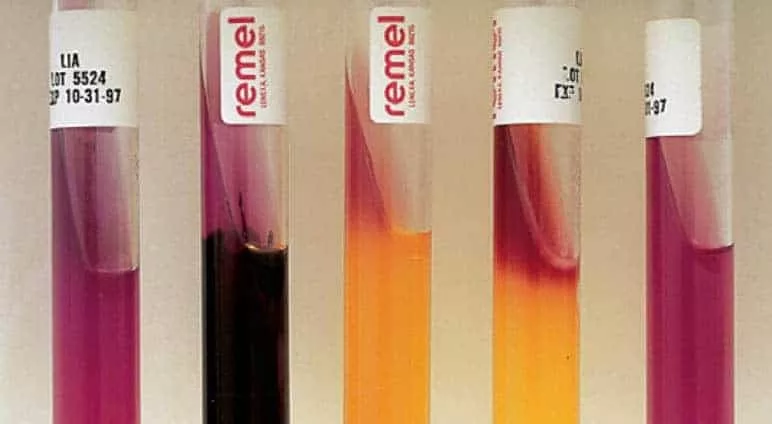
Aim – To prepare Nutrient agar stab and slant Requirement – Test tube, Inoculation loop, cotton plugs, Nutrient agar media, Pipette, Water bath, Autoclave, Glass rod, Laminar air flow, Incubator Theory – A test tube containing sterilised agar medium is…
In reference to the letter of Mission Director, NIIM & Executive Director, WBSH&FW Samiti, vide memo no. IIFW/NUIIM-454/2019/716(2) dated 15/02/2023. DH&FWS and Office of the CMOH. Purba Bardhaman is inviting online Applications for engagement (on contractual basis) of Pharmacist for…
BRIDGEWATER, N.J.–(BUSINESS WIRE)– Amneal Pharmaceuticals, Inc. (NYSE: AMRX) today announced the U.S. Food and Drug Administration (FDA) has accepted for review the Abbreviated New Drug Application (ANDA) for naloxone hydrochloride nasal spray, USP, 4mg, which is the generic version of Narcan® and…