Govt Pharmacist Vacancy 2024 National Health Mission Delhi – South East District – Apply now for a contractual pharmacist position under NHM.
Category: M Pharma Jobs
Find advanced opportunities with an M Pharma degree in research, development, and higher-level pharmaceutical roles.
Pharmacy Jobs for D Pharm B Pharm M Pharm and PhD Students: Explore exciting job opportunities across various institutes and eligibility details!
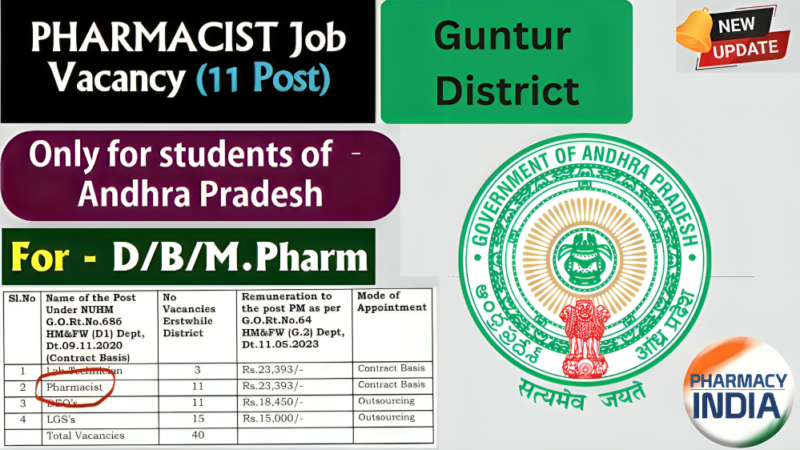
Pharmacist Jobs in Guntur District | 11 Posts | For D.Pharm/B.Pharm/M.Pharm graduates | (Andhra Pradesh Only)s! Apply offline by October 30. Don’t miss out!
RSMSSB Exam Calendar 2024-25 Out for Various Posts! Download PDF, check exam dates and schedule for Pharma Assistant and other vacancies.
Pharmacist Recruitment by Transfer (Now Absorption) at HQ A&N, Indian Navy. Explore exciting job opportunities in the Navy!
PM Internship Scheme 2024 for Pharma Students – Details & Benefits: Discover internship opportunities, eligibility, and application steps!
MPSC Drug Inspector Recruitment 2024: Get complete details on eligibility, syllabus, exam pattern, and application process for 87 Group-B posts.
Walk-In for M.Pharm/B.Pharm at Alembic Pharmaceuticals: Join a leading pharma company and advance your career today!
Junior Scientist (M Pharma) Vacancies at NIB – Exciting opportunity for M.Pharm graduates! Apply by September 23, 2024, for a rewarding career.
Walk-in Interview for Assistant Professor at SPER Jamia Hamdard – Join as Assistant Professor in Pharmaceutical Chemistry at a leading university.