ICH, or the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, is what the acronym stands for. ICH is a collaborative effort in which regulators from Europe, Japan, and the United States participate in scientific and technical talks about the testing techniques and their evaluation to guarantee the safety, quality, and efficacy of the medicines. The European Union started the process of harmonising regulatory requirements in 1980.
Negotiations between Europe, Japan, and other countries took held bilaterally in Paris in 1989 U.S. at WHO summit on drug regulatory issues discussing potential for harmonisation authority to put particular action plans into effect. Officials went to the IFPMA (International Federation of Pharmaceutical Associations), and they talked about a cooperative industry-regulatory endeavour on International harmonisation was seen, and ICH followed. ICH was created in April 1990 at a meeting held by the EFPIA (European Federation of At Brussels (Pharmaceutical Industries and Association).
Process of ICH
The International Conference on Harmonization (ICH) was founded in 1990 as a combined governmental and industry endeavour to streamline the process for creating and registering new pharmaceuticals in Europe, Japan, and the United States and to make them quickly available to patients. The ICH fulfils the following functions.
1) It keeps track of, updates, and strengthens the harmonisation of technical criteria on a global scale.
2) It guarantees the quality, safety, and efficacy of medications that must be created and registered in a time- and money-efficient manner.
3) From a global standpoint, it promotes and defends public health.
4) It avoids pointless repetition of human clinical trials.
5) It lessens animal experimentation without sacrificing its effectiveness or safety.
6) It increases the effectiveness of drug development over the world.
Participants of ICH
Representatives from six parties, including the regulatory agencies and research-based companies in the United States, Japan, and the European Union, make up the ICH steering committee and its subgroups. The ICH observers have been working with the ICH process to connect the non-ICH nations since the beginning. The World Health Organization (WHO), Canada, and the European Free Trade Association (EFTA) are the non-voting members of the ICH steering committee and its sub-group.
Process of Harmonization
- The ICH steering committee is in charge of ICH administration, which includes choosing whether to approve a new issue, maintain an existing guideline, or carry out a specific implementation task.
- Every harmonisation initiative begins with a concept paper that contains a synopsis of the idea. Depending on the type of harmonisation effort, a business plan may also be required.
- Any observer or ICH member may suggest a fresh ICH implementation activity. The ICH steering committee approves the formation of an EWG/IWG and decides which ICH tasks will be adopted.
- The formal ICH procedure, questions and answers procedure, revision procedure, and maintenance procedure are the categories under which the ICH harmonisation activities are grouped.
Formal ICH Procedure
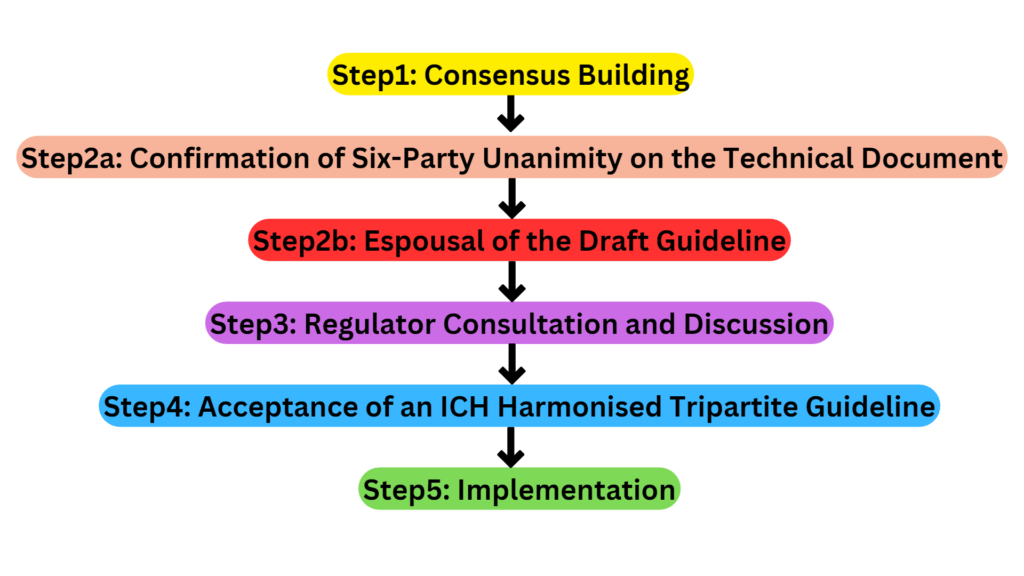
Revision Procedure
The revision process is created when an existing ICH guideline’s scientific or technical content is shown to be false, or when new data must be included without altering the existing ICH guideline. In the second scenario, the fresh information can be annexed or included as an addition to the relevant guideline. The process begins with the steering committee’s approval of a concept paper. For modifications, a business strategy is not necessary. There is established an EWG with the membership outlined in the concept paper.
The main difference between the revision process and the formal ICH procedure’s five steps is that the end result is a revised version of an existing guideline rather than a brand-new one. A guideline’s revision is indicated by appending the letter R1 to the end of its customary designation. A document is given a new designation at each revision when a guideline is amended more than once, such as R2, R3, or R4. If an annexure or addendum has been created, it is incorporated into the existing guideline at Step 4 to create an updated guideline.
Maintenance Procedure
The Q3C guideline on impurities: residual solvents can only be changed, in which case the maintenance method is applicable. When new information needs to be added or the technical content needs to be updated, this procedure is employed.
QSEM
ICH Multidisciplinary Guidelines



