IUPAC nomenclature
(R)-3-[-1-hydroxy-2-(methylamino)ethyl]phenol.
Physicochemical Properties
Chemical Formula: C9H13NO2
Molecular Weight: 167.21 g/mol
Chemical Structure: Phenylephrine is a phenethylamine that contains a phenyl ring and an ethanolamine side chain. It is the (R)-enantiomer of pseudoephedrine.
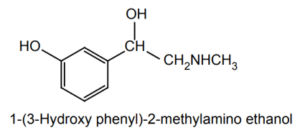
Presence of ring: Benzene ring
Number of chiral centers: 1
Physical State
Physical Form: Phenylephrine is typically found as a white or nearly white, odorless crystalline powder.
Solubility: Phenylephrine is soluble in water and ethanol.
Melting Point: Phenylephrine hydrochloride, which is a common salt form of phenylephrine, has a melting point of about 140-145 °C (284-293 °F).
pKa: The pKa of phenylephrine is around 9.5, indicating that it is a weak base.
Stability: Phenylephrine hydrochloride is stable under normal conditions but may degrade upon exposure to light, air, or moisture. Proper storage in airtight containers and protected from light is necessary to maintain its stability.
SAR of Phenylephrine
1. Phenyl Ring: Phenylephrine contains a phenyl ring, which is essential for its activity. The presence of the phenyl ring is crucial for binding to adrenergic receptors, specifically α-adrenergic receptors.
2. Ethylamine Group: Phenylephrine has an ethylamine group (a side chain) attached to the phenyl ring. This group is important for the compound’s adrenergic receptor binding activity. It provides the necessary stereochemistry and spatial arrangement for proper interaction with the receptor sites.
3. Hydroxyl Group: Phenylephrine has a hydroxyl (-OH) group in the para position (fourth carbon) on the phenyl ring. This hydroxyl group is essential for hydrogen bonding interactions, which are crucial for receptor binding. It enhances the affinity of the compound for adrenergic receptors.
4. Stereochemistry: The stereochemistry of phenylephrine is crucial for its activity. Phenylephrine is the (R)-enantiomer of the compound. The (S)-enantiomer, known as pseudoephedrine, has different pharmacological properties. The (R)-enantiomer is responsible for the desired decongestant effects.
5. Lack of Substituents: Phenylephrine lacks significant substituents on the phenyl ring. Substituents in various positions can drastically alter the compound’s activity. In the case of phenylephrine, the absence of bulky substituents allows it to efficiently bind to adrenergic receptors.
6. Selectivity for α-Adrenergic Receptors: Phenylephrine exhibits selectivity for α-adrenergic receptors, particularly the α1 subtype. This selectivity is crucial for its vasoconstrictive effects, which help in reducing nasal congestion.
Mechanism of action
a. An alpha-1 adrenergic agonist, phenylephrine causes mydriasis and vasoconstriction. Those factors rely on the administration’s venue and route.
b. By irritating alpha-1 adrenergic receptors throughout the body, the medication causes a rise in systolic and diastolic pressure as well as peripheral vascular resistance.
c. The vagus nerve is stimulated by the rise in blood pressure, which leads to reflex bradycardia.
Uses
1. Haemorrhoids
2. Hypotension
3. Priapism







