Acetazolamide IUPAC Nomenclature, SAR, Physicochemiscal properties, Synthesis, Mechanism of Action and Uses. In our comprehensive guide, unravel the synthesis of this compound and explore the structure-activity relationships (SAR) of Acetazolamide. Understand the pivotal role played by its sulfonamide group, aromatic ring, nitrogen atoms, steric factors, electronegativity, functional groups, and isosteric replacements in shaping its pharmaceutical properties.
IUPAC Nomenclature
N-(Sulphonamido-1,3,4-thiadiazol-2-yl) acetamide
Chemical Structure
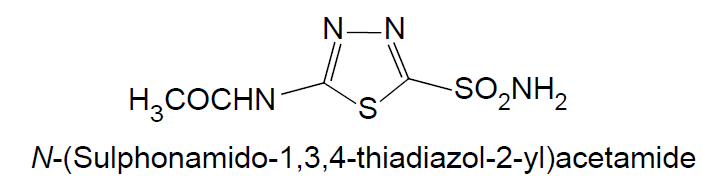
Physicochemical Properties
1. Molecular formula: C4H6N4O3S2
2. Physical Appearance: Acetazolamide is a white to faintly yellowish-white crystalline powder. It is odorless and has a bitter taste.
3. Solubility: Acetazolamide is sparingly soluble in water and slightly soluble in alcohol and chloroform.
4. Melting Point: The melting point of acetazolamide is reported to be around 256-260°C.
5. Molecular Weight: 222.25 g/mol.
6. Acidity (pKa): Acetazolamide is a weak acid. It has two pKa values: around 7.2 and 10.3.
7. Partition Coefficient (Log P): The logarithm of the octanol/water partition coefficient (Log P) for acetazolamide is around -0.82, indicating that it is more soluble in water than in octanol.
Synthesis
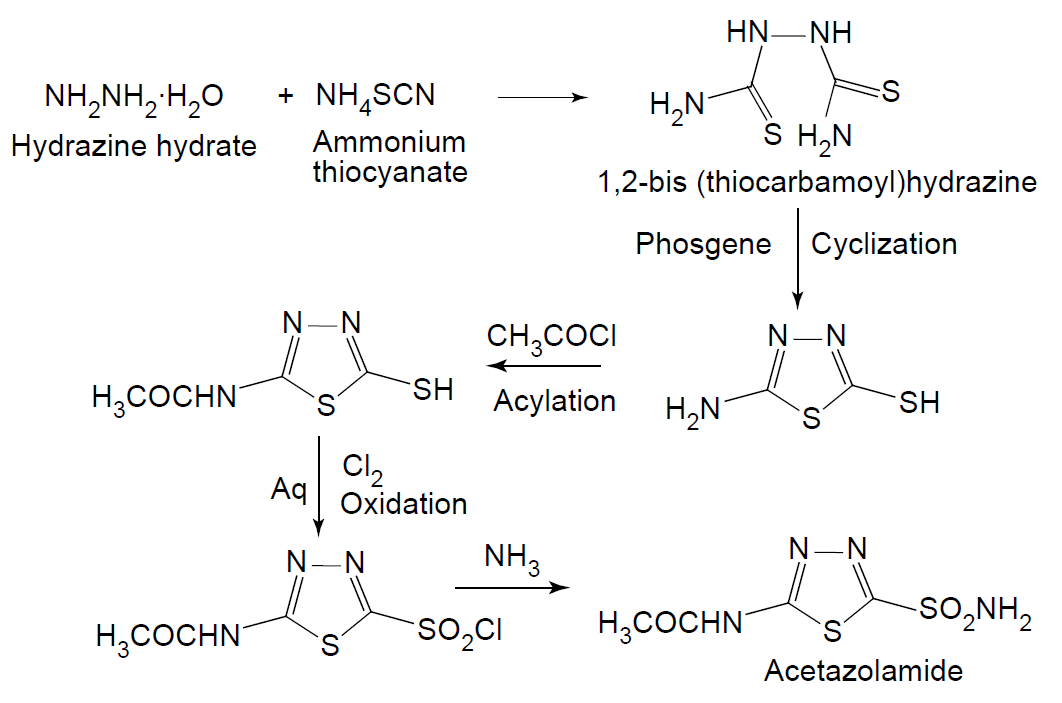
SAR of Acetazolamide
1. Sulfonamide Group: Acetazolamide contains a sulfonamide group (-SO2NH2), which is essential for its carbonic anhydrase inhibitory activity. This group interacts with the active site of the enzyme, forming strong hydrogen bonds. Substituting or modifying this group can significantly affect the drug’s activity.
2. Aromatic Ring: Acetazolamide has an aromatic ring in its structure, which is important for its binding affinity to the carbonic anhydrase enzyme. Modifications to this ring can influence the drug’s potency and selectivity.
3. Nitrogen Atoms: The presence of nitrogen atoms in the structure of acetazolamide is crucial for its activity. Nitrogen atoms participate in hydrogen bonding and electrostatic interactions with the enzyme, enhancing the drug’s binding affinity.
4. Steric Factors: The spatial arrangement of substituents around the acetazolamide molecule plays a role in its activity. Steric hindrance caused by bulky substituents can affect the drug’s ability to bind to the enzyme’s active site.
5. Electronegativity: The electronegativity of atoms in the molecule, particularly oxygen and sulfur, influences the strength of hydrogen bonding interactions. This can impact the drug’s affinity for the enzyme.
6. Functional Groups: Various functional groups in acetazolamide, such as amines and amides, contribute to its overall pharmacological activity. Modifying these functional groups can lead to changes in the drug’s properties, including solubility and stability.
7. Isosteric Replacements: Certain atoms or groups in the acetazolamide molecule can be replaced with structurally similar atoms or groups (isosteric replacements) without losing activity. This knowledge can be used in drug design to optimize the compound’s properties.
Mechanism of action
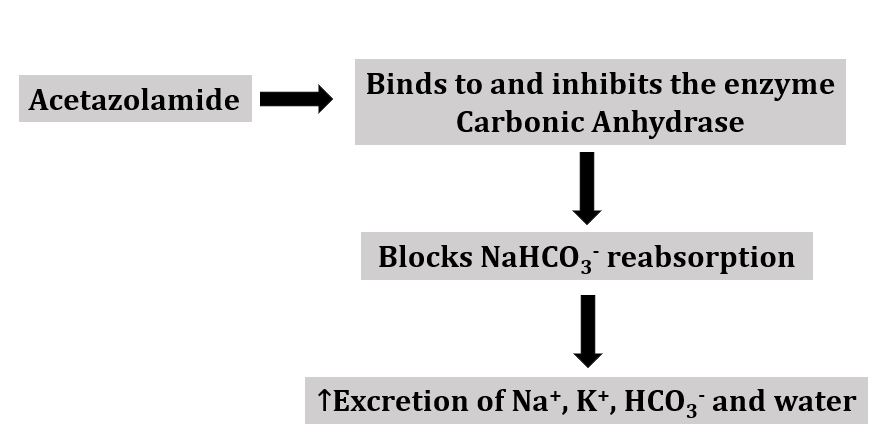
Uses
1. Glaucoma
2. Alkalinization of urine
3. Metabolic alkalosis
4. Mountain sickness
5. Epilepsy
6. Hyperphosphataemia
Methotrexate: SAR, Synthesis, Mechanism, and Uses
Promethazine HCl SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses
Cimetidine SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses







