Mechlorethamine IUPAC Nomenclature, SAR, Physicochemiscal properties, Synthesis, Mechanism of Action and Uses. Mechlorethamine, a powerful anticancer agent known for its bis(2-chloroethyl)amine structure. Discover its intricate IUPAC nomenclature as 2-Chloro-N-(2-chloroethyl)-N-methylethan-1-amine, revealing a structure where two chlorine atoms bind to a nitrogen atom, forming a potent alkylating agent.
IUPAC nomenclature
2-Chloro-N-(2-chloroethyl)-N-methylethan-1-amine
Chemical Structure
Its structure consists of two chlorine atoms attached to a nitrogen atom, forming a bis(2-chloroethyl)amine structure.

Physicochemical Properties
1. Chemical formula: C5H11Cl2N.
2. Molecular Weight: 156.06 g/mol.
3. Melting Point: Mechlorethamine is a solid at room temperature. The exact melting point can vary, but it typically melts around 28-30°C (82-86°F).
4. Solubility: Mechlorethamine is soluble in water and many organic solvents. This solubility allows it to be administered intravenously or topically, depending on the medical application.
5. Stability: Mechlorethamine is sensitive to light, heat, and moisture. It is typically stored as a powder or in a stable formulation to prevent degradation.
6. pKa (Acid Dissociation Constant): The pKa value of mechlorethamine is not readily available in commonly referenced sources. However, being a tertiary amine, it would have basic properties and can act as a weak base.
7. Octanol/Water Partition Coefficient (LogP): The LogP value represents the lipophilicity of a compound, indicating its tendency to partition between octanol (lipid) and water (hydrophilic). This value is crucial for understanding a drug’s absorption, distribution, and elimination. The LogP value for mechlorethamine is not widely reported but is likely to be low given its polar nature.
SAR of Triprolidine
1. Alkylating Group: The alkylating activity of mechlorethamine is due to the presence of the bis(2-chloroethyl)amine group. This group contains two chloroethyl moieties (-CH2CH2Cl) that are highly reactive. These groups are essential for forming covalent bonds with nucleophilic sites on DNA, such as the nitrogen atoms of purine bases (adenine and guanine). This alkylation interferes with DNA replication and transcription, leading to cell death.
2. Reactivity of Chloroethyl Groups: The chlorine atoms in the chloroethyl groups increase the electrophilicity of the adjacent carbon atoms, making them highly reactive. This reactivity allows mechlorethamine to readily form covalent bonds with nucleophilic centers in DNA molecules.
3. Bifunctional Nature: Mechlorethamine is bifunctional, meaning it has two reactive sites. Bifunctional alkylating agents are capable of cross-linking two different DNA strands or forming intra-strand cross-links within a single DNA molecule. This cross-linking inhibits the separation of DNA strands, further disrupting cellular processes like replication and repair.
4. Aqueous Stability: Mechlorethamine is unstable in aqueous solutions. Its reactivity with water can lead to the formation of degradation products. While this instability can be a challenge in formulation and storage, it is also a critical aspect of its mechanism of action. The drug needs to be activated in the body to exert its cytotoxic effects.
5. Cellular Targets: The primary target of mechlorethamine is DNA. Its ability to form covalent bonds with DNA bases disrupts the normal functioning of the genetic material. This interference with DNA structure and function triggers cellular responses, including apoptosis (programmed cell death), leading to the elimination of cancer cells.
Synthesis
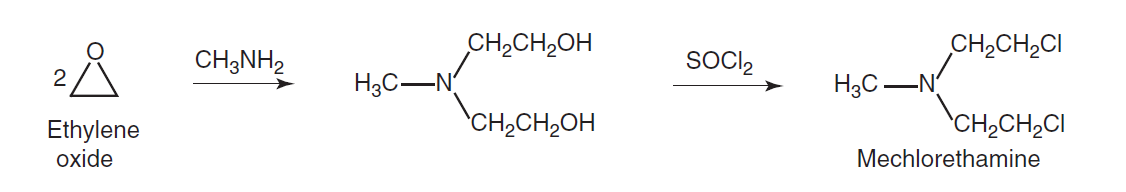
Mechanism of action
1. Mechlorethamine forms highly reactive carbonium ion intermediates that react with strong nucleophilic substituents (e.g., phosphate, amino, –SH, –OH, – COOH and imidazole groups) to form covalent bonds, thus alkylating the target molecule.
2. Guanine in DNA is very susceptible at its N-7 position.
3. Therefore, alkylation of guanine results in cross-linking of DNA strands, linking of DNA to a closely related protein, base pairing of guanine with thymine (and not with cytosine), or breakage of DNA strand.
Uses
1. It is used for the treatment of stages III and IV of Hodgkin’ s disease, lymphosarcoma, chronic myelocytic or chronic lymphocytic leukaemia, polycythemia vera, mycosis fungoides, and bronchogenic carcinoma.
2. It is also used for the treatment of metastatic carcinoma.
Acetazolamide: SAR, Synthesis, Mechanism, and Uses
Methotrexate: SAR, Synthesis, Mechanism, and Uses
Promethazine HCl SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses
Cimetidine SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses
Download B. Pharma 1st Semester Model Papers
B. Pharma 4th Semester Model Papers
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
Click here to follow us on Instagram
Click here to Join our Telegram Channel
Download PHARMACY INDIA MOBILE APP from Google Play Store



