Unlock the secrets to mastering the GPAT exam and catapult your career in pharmacy! Dive into the ultimate resource: the GPAT 2023 Previous Year Question Paper, now accessible on pharmacyindia.co.in. Elevate your preparation with this comprehensive guide, meticulously designed to enhance your understanding of key concepts and exam patterns.
Gain invaluable insights into the structure of the GPAT exam, refine your strategies, and boost your confidence for success. Don’t miss out on this invaluable tool as you embark on your journey towards acing the GPAT exam and unlocking boundless opportunities in the pharmaceutical realm. Visit pharmacyindia.co.in today and pave the way for your triumph!
PHARMACEUTICS
1. The addition of Monobasic Potassium Phosphate to the suspended Bismuth Subnitrate particles cause the A to B owing to the C
(a) A- negative zeta potential, B-decrease, C- adsorption of the negatively charged phosphate anion
(b) A-positive zeta potential, B-increase, C- adsorption of the negatively charged phosphate anion
(c) A-positive zeta potential, B-decrease, C- adsorption of the negatively charged phosphate anion
(d) A- positive zeta potential, B-decrease, C- adsorption of the positively charged hydrogen anion
2. Noyes-Whitney equation predicts
(a) An increase of dissolution rate if the particle size is reduced by micronization because of an increase in area
(b) Relationship between the radius of the diffusing molecule and its diffusion coefficient
(c) The influence of electrolyte on the rate constant
(d) An equilibrium between the surfactant and the drug molecules at the surface of the solution and in the bulk of the solution
3. Which type of in-vitro-in-vivo correlation compares % drug released Vs % drug absorbed
(a) Level C
(b) Level A
(c) Multiple level C
(d) Level B
4. Ideally BA studies should be carried on _______ volunteers
(a) Aged
(b) Children
(c) Healthy
(d) Patient
5. Given below are two statements, one labelled as Assertion (A) and the other labelled as Reason (R)
Assertion (A) :In case of Salicylic Acid Ointment BP Wool Alcohol Ointment made with white soft paraffin is used
Reason (R) : Wool Alcohol Ointment made with white soft paraffin is used because the medicament is coloured
In the light of the above statements, choose the most appropriate answer from the options given below
(a) Both (A) and (R) are true and (R) is the correct explanation of (A)
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A)
(c) (A) is true but (R) is false
(d) (A) is false but (R) is true
Click Here to join WhatsApp Group
6. If S is the solubility of small particles of radius r, is the normal solubility (i.e., of a solid consisting of fairly large particles), is the interfacial energy, M is the molecular weight of the solid, ρ is the density of the bulk solid, R is the gas constant and T is the thermodynamic temperature, then which of the following equation indicates the changes in interfacial free energy that accompany the dissolution of particles of varying sizes causing the solubility of substance to increase with decreasing particle size
(a) Log (So/S)=2γMr/2.303RTρ
(b) Log (S/So )=2γMr/2.303RTρ
(c) Log (So/S)=2γMr/2.303RTρr
(d) Log (S/So )=2γM/2.303RTρr
7. Indicate which of the following molecular characteristics will be expected to increase the solubility of a simple solute in an aqueous solution
(a) A high melting point
(b) The presence of polar group
(c) A high molecular surface area
(d) A high boiling point
8. Match List I with List II
| LIST- (DISSOLUTION APPARATUS) | LIST-II (NAME) |
| 1. Type 1 | [P] Reciprocating holder |
| 2. Type 5 | [Q] Paddle over disk |
| 3. USP App 6 | [R] Basket type |
| 4. USP App 7 | [S] Cylinder apparatus |
Choose the correct answer from the options given below
(a) 1-[R], 2-[Q], 3-[S], 4-[P]
(b) 1-[S], 2-[P], 3-[Q], 4-[R]
(c) 1-[Q], 2-[R], 3-[P], 4-[S]
(d) 1-[P], 2-[Q], 3-[R], 4-[S]
9. A clear, sweetened hydroalcoholic liquid containing medicament is known as
(a) Elixir
(b) Syrup
(c) Tincture
(d) Decoction
10. Match List I with List II
| LIST-I (NAME OF EMULSIFIER ) | LIST – II (REMARK) |
| 1. Triethanolamine oleate | [P] Surface active agent (non-ionic) |
| 2. N-cethyl N-ethyl morpholinium ethosulfate (Atlas G-263) | [Q] Hydrophilic colloid |
| 3. Polyoxyethylene sorbitan mono oleate (Atlas Tween 80) | [R] Surface- active agent (anionic) |
| 4. Gelatin | [S] W/O Emulsifier (HLB= 4.3) |
| [T] Surface active agent (cationic) |
Choose the correct answer from the options given below:
(a) 1-[Q], 2-[S], 3-[T], 4-[R]
(b) 1-[R], 2-[T], 3-[P], 4-[Q]
(c) 1-[T], 2-[S], 3-[Q], 4-[R]
(d) 1-[S], 2-[R], 3-[T], 4-[P]
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
11. Which of the following equipment is based on the principle of Pohlman liquid whistle
(a) Ultrasonifier
(b) Mechanical stirrer
(c) Silverson homogeniser
(d) Colloid mill
12. Which of the following is the correct choice of particle size measurement technique in scoring order of size
[P] Sieve [Q] Anderson Pipette [R] Coulter counter [S] Light scattering
(a) P, Q, R, S
(b) Q, S, R, P
(c) P, R, Q, S
(d) S, P, R, Q
13. Which one of the following is an example of ointment prepared by trituration and containing liquids and solids
(a) Salicylic and Sulphur Ointment BPC
(b) Whitfield’s Ointment BPC
(c) Hamamelis Ointment BPC
(d) Resorcinol Ointment Compound BPC
14. ‘Picking’ is a term used to describe
(a) Separation of tablet into two or more layers
(b) The situation when the surface material from a tablet that is sticking to and being re moved from the tablet’s surface by a punch
(c) Unequal distribution of colour on a tablet
(d) Partial or complete separation of the top and bottom crowns of a tablet from the main body of the tablet
15. Which one of the following is an example of a chelate
(a) Cisplatin
(b) Hemoglobin
(c) Iodine
(d) Ferrocene
Click Here to Join Telegram Group
16. A 2.0% saline solution is
(a) Hypotonic
(b) Hypertonic
(c) Isotonic
(d) Iso-osmotic
17. Which of these is not a colligative property
(a) Osmotic pressure
(b) Depression of freezing point
(c) Elevation in boiling point
(d) Polymorphism
18. Sarong SpA semiautomatic equipment is used for the
(a) Filling and packaging line for topical pharmaceutical aerosols
(b) Filling of hard gelatin capsule
(c) Production of suppositories
(d) Inserting rubber closure in vials
19. In a mechanical model of a viscoelastic material, showing both viscosity of liquid state and elasticity of solid state combined in series is termed as
(a) Voigt Element
(b) Creep Element
(c) Maxwell Element
(d) Retardation Element
20. Which of the following factors affect the heat of reaction based on Kirchoff equation
(a) Molecularity
(b) Temperature
(c) Pressure
(d) Volume
Download PHARMACY INDIA MOBILE APP from Google Play Store
21. Rittinger’s hypothesis relates
[P] Energy used in size reduction [Q] New surface area produced
[R] Equivalent shape [S] Reynold’s number
(a) P and Q
(b) Q and S
(c) P and R
(d) P and S
22. Which of the following ICH Harmonized Tripartite Guidelines related to stability, provides the general requirements for stability testing of new drug substances and products
(a) Q1A (R2)
(b) Q1B
(c) Q1D
(d) Q1E
23. Given below are two statements
Statement I :Rubber stoppers cannot withstand Pyrogen-destructive temperatures Statement II : In case of rubber stopper for injections reliance must be on an effective sequence of washing, thorough rinsing with WFI, prompt sterilization and protective storage to ensure adequate pyrogen control
In the light of the above statements, choose the most appropriate answer from the options given below
(a) Statement I and Statement II are correct
(b) Statement I and Statement II are incorrect
(c) Statement I is correct but Statement II is incorrect
(d) Statement I is incorrect but Statement II is correct
24. Match List I with List II
| LIST – I (PART OF THE VALUE ASSEMBLY ) | LIST- II (PURPOSE OF PARTS) |
| 1. Gasket | [P] Links the dip tube and the stem and the actuator |
| 2. Spring | [Q] Prevents the leakage |
| 3. Mounting cup | [R] Holds the Gasket in place |
| 4. Housing | [S] Holds the valve in place |
Choose the correct answer from the options given below
(a) 1-[R], 2-[Q], 3-[S], 4-[P]
(b) 1-[Q], 2-[R], 3-[S], 4-[P]
(c) 1-[S], 2-[P], 3-[Q], 4-[R]
(d) 1-[R], 2-[P], 3-[S], 4-[Q]
Click Here to join WhatsApp Group
25. Absolute solubility does not rely on standard condition of
(a) pH
(b) Pressure
(c) Temperature
(d) Volume
26. Vanishing cream is an ointment that may be classified as
(a) Water soluble base
(b) Oleaginous base
(c) Absorption base
(d) Emulsion base
27. In terms of the kinetics degradation in suspension is
(a) First order
(b) Second order
(c) Pseudo zero order
(d) Zero order
28. While preparing the following
Rx
Salicylic acid : 3g
Sulfur ppt : 7g
Lanolin : 10g
White petroleum : 10g
The pharmacist should:
(a) Use a rubber spatula to weigh and levigate the salicylic acid
(b) Mix the powders using geometric dilution in a motar
(c) Place on an ointment tile and levigate the ingredients using geometric dilution
(d) All of the above
29. EDTA is an example of
(a) Unidentate Ligand
(b) Bidentate Ligand
(c) Tridentate Ligand
(d) Hexadentate Ligand
30. Efficiency of an reversible engine is given by
(a) Clapeyron equation
(b) Clausius Clapeyron equation
(c) Gibbs-Helmholtz equation
(d) Carnot theorem
Follow us on Instagram
31. A sample of glucose was decomposed at 140° C in a solution containing 0·030 M HCl. The velocity constant, k, was found to be 0·0080 hr-1. If the spontaneous rate constant, is 0·0010 hr-1 and the catalysis due to hydroxyl ions in this acidic solution is considered as negligible, then the catalytic coefficient, kH is
(a) 0·22 per mole per hour
(b) 0·233 per mole per hour
(c) 0·27 per mole per hour
(d) 0·29 per mole per hour
32. Which of the following characteristics is most likely to be associated with a high apparent volume of distribution
(a) Penetration across the blood brain and blood testis barriers
(b) Extensive binding to plasma protein
(c) Distribution into total body water
(d) Extensive binding to tissue constituents
33. Which of the following drugs does not bind to haemoglobin
[P] Chlorpromazine
[Q] Phenobarbital
[R] Phenothiazine
[S] Phenytoin
Choose the most appropriate answer from the options given below
(a) Q, R and S only
(b) Q and R only
(c) R and S only
(d) P only
34. The more appropriate purpose of spiral scrapper in Swenson-Walker crystallizer is
(a) Agitation of sample (solution)
(b) Conveying the crystals
(c) Prevent an accumulations of crystals on the cooling surface
(d) Provides desired temperature to the sample (solution)
35. If u is velocity of fluid, ρ is density of fluid, L is length of the pipe, D is diameter of the pipe, f is friction factor and ΔP1 is pressure drop, then the equation ΔP1 = (2fu²Lρ) ÷ D represents
(a) Hagen-Poiseuille equation
(b) Bernoulli equation
(c) Fanning’s equation
(d) Reynolds equation
Click Here to Join Telegram Group
36. Schedule ‘O’ governs the standards for
(a) Disinfectant fluids
(b) List of equipment to run a pharmacy
(c) Life period (expiry) of drugs
(d) Manufacturing and analytical records of drugs
37. The number of persons elected as the member of the Pharmacy Council of India from the teaching profession is
(a) Five
(b) Six
(c) Seven
(d) Eight
38. The fluid flows through the filter medium by virtue of
(a) Pressure difference across the filter
(b) Temperature difference across the filter
(c) Volume difference across the filter
(d) Potential difference across the filter
39. Specific requirements for manufacture of sterile products and parenteral preparations are prescribed in which of the following part of the Schedule-M
[DROPPED QUESTION]
(a) PART-IV
(b) PART-IB
(c) PART-IC
(d) PART-ID
40. Identify the schedule for which the following cautionary labelling is mandatory as per Drugs and Cosmetics
[DROPPED QUESTION]
(a) Use within one month of opening
(b) Name and concentration of preservative
(c) Not for injection
(d) If irritation persists or increases, discontinue use and consult physician. Keep container tightly closed
41. Para aminohippuric acid (PAH) clearance test is employed to measure
(a) Renal blood flow
(b) Liver blood flow
(c) Cerebral blood flow
(d) Venous blood flow
PHARMACEUTICAL CHEMISTRY
42. Alkyl group in Grignard reagent serve as
(a) Carbene
(b) Free radical
(c) Aromatic carbocation
(d) Carbanion
43. Which of the following compound would be expected to have greatest florescence
 44. Conversion of cyclic ketone to ring expended cyclic ester takes place by
44. Conversion of cyclic ketone to ring expended cyclic ester takes place by
(a) Willgerodt rearrangement
(b) Michael rearrangement
(c) Lossen rearrangement
(d) Baeyer Villiger rearrangement
45. Identify A, B and C in below reaction
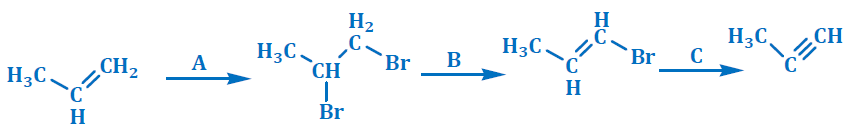 (a) A=Br2, B=KOH, C=NaNH2
(a) A=Br2, B=KOH, C=NaNH2
(b) A=Br2, B=HCl, C=NaNH2
(c) A =Br2, B = HCl, C = NaBH4
(d) A = Br2, B = KOH, C = NaBH4
46. Identify intermediate forms in following substitution reaction
 (a) Cyclohexa-1,3-dien-4-yne
(a) Cyclohexa-1,3-dien-4-yne
(b) Cyclohexa-1,3-dien-5-yne
(c) Cyclohexa-1,4-dien-5-yne
(d) Cyclohexa-1,5-dien-3-yne
Click Here to join WhatsApp Group
47. Match List I with List II
| LIST – I (NAME OF THE DRUG) | LIST-II (CHEMICAL CLASS) |
| 1. Zolpidem | [P] Cyclopyrrolone |
| 2. Zaleplon | [Q] Benzodiazepine |
| 3. Zopiclone | [R] Imidazopyridine |
| 4. Triazolam | [S] Pyrazolopyrimidine |
Choose the correct answer from the options given below
(a) 1-[S], 2-[R], 3-[Q], 4-[P]
(b) 1-[Q], 2-[S], 3-[R], 4-[P]
(c) 1-[R], 2-[S], 3-[P], 4-[Q]
(d) 1-[P], 2-[R], 3-[S], 4-[Q]
48. Addition of HBr to 1,3-butadiene at 40°C yields
(a) 80% 1,4-addition product and 20% 1,2-addition product
(b) 80% 1,2-addition product and 20% 1,4-addition product
(c) 80% 1,2-addition product and 20% 1,3-addition product
(d) 80% 1,2-addition product and 20% 1,4-addition product
49. Which heterocyclic ring is fused to a steroidal nucleus in Danazol
(a) Thiazole
(b) Isoxazole
(c) Imidazole
(d) Pyrazole
50. Which of the following factor make carbonyl group in acyl compounds too susceptible to nucleophilic attack
(a) The tendency of oxygen to acquire electrons even at the expense of gaining positive charge
(b) The tendency of oxygen to acquire electrons even at the expense of gaining negative charge
(c) The tendency of carbon to loose electrons even at the expense of gaining negative charge
(d) The tendency of carbon to loose electrons even at the expense of gaining positive charge
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
51. Phenol reacts with chloroform in presence of aqueous sodium hydroxide to give chief product
(a) 2-Chloro Benzaldehyde
(b) 2-Hydroxy Benzaldehyde
(c) 3-Hydroxy Benzaldehyde
(d) 3-Chloro Benzaldehyde
52. Replacement of the diazonium group by halogen in presence of copper powder is
(a) Sandmeyer reaction
(b) Gattermann reaction
(c) Hofmann reaction
(d) Gabriel reaction
53. Identify pair of C4 epimers
(a) D-glucose and D-galactose
(b) D-glucose and D-fructose
(c) D-glucose and D-mannose
(d) D-glucose and D-xylulose
54. which is an example of aromatic nucleophilic substitution reaction
(a) Chichibabin
(b) Gattermann Koch reaction
(c) Kolbe’s reaction
(d) Friedel-Crafts reaction
55. Amphetamine undergoes one of the following metabolic reaction to convert to 1- phenyl-2-propanol metabolite via ketone formation
(a) Hydrolysis
(b) Oxidation
(c) Reduction
(d) Hydroxylation
Click Here to Join Telegram Group
56. What is the popular common name for a bioactive compound with chemical name of (m-hydroxy phenyl) trimethyl ammonium methyl sulphate dimethyl carbamate
(a) Neostigmine
(b) Pyridostigmine
(c) Physostigmine
(d) Metastigmine
57. Which among the following Cephalosporins has an unusual 5-thio-1,2,3,4-tetrazole substituent attached to core heterocyclic nucleus through a methylene bridge
(a) Cefazolin
(b) Cefamandole
(c) Cefoxitin
(d) Cefadroxil
58. Which one of the following molecules has a dipole moment
(a) CS2
(b) CHCl3
(c) CH3
(d) CO2
59. The C-2 epimer of D-glucose is
(a) D-Mannose
(b) L-Fructose
(c) D- Glucopyranose
(d) L-Arabinose
60. Which of the following is correct order of stability of free radicals
(a) 3°>2°>1°>CH3°>allyl
(b) 3°>2°>1°>allyl>CH3°
(c) CH3°>3°>2°>1°>allyl
(d) Allyl>3°>2°>1°>CH3°
Download PHARMACY INDIA MOBILE APP from Google Play Store
61. Isoquinoline on treatment with oleum at 90 °C yields majorly
(a) Isoquinoline-3-sulfonic acid
(b) Isoquinoline-5-sulfonic acid
(c) Isoquinoline-6-sulfonic acid
(d) Isoquinoline-7-sulfonic acid
62. Eicosanoids are polyunsaturated fatty acids of___carbons
(a) 30 (b) 25 (c) 15 (d) 20
63. Conjugation of a drug includes the following EXCEPT
(a) Glucuronidation
(b) Sulfate formation
(c) Hydrolysis
(d) Methylation
64. The potential of the calomel electrode depends upon
(a) The concentration of potassium chloride solution
(b) Concentration of mercuric chloride
(c) Concentration of mercury
(d) Membrane
65. When exposed to carbon monoxide, the base pigment of Cytochrome P enzymes absorb light at
(a) 450 nm (b) 370 nm (c) 254 nm (d) 600 nm
66. In atomic absorption spectroscopy, back ground correction performed using a single hol low cathode lamp pulsed first with a low current and then with a high current is called
(a) Smith Hieftje background correction
(b) Continuous source background correction
(c) Zeeman effect background correction
(d) Hollow cathode background correction
67. Predict the theoretical max value for the following compound using Woodward-Fieser rules. Base value for the compound is 215 nm
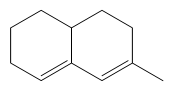 (a) 240 nm (b) 220 nm (c) 225 nm (d) 235 nm
(a) 240 nm (b) 220 nm (c) 225 nm (d) 235 nm
68. All the following about back titration are true, EXCEPT
(a) It is performed when the rate of reaction between the analyte and reagent is fast
(b) It is performed when the rate of reaction between the analyte and reagent is slow
(c) It is performed when the standard solution lacks stability
(d) It is the process in which excess of standard solution used to react with an analyte is determined by titration with a second standard solution
69. A chromatogram of a peak provided a retention time at 5.4 minutes and 0.41 base width of the peak. The number of plates or the peak obtained is
(a) 210.7 (b) 173 .5 (c) 78.4 (d) 2775.5
70. In UV spectrophotometer, lamp used to generate UV spectrum is
(a) Tungsten
(b) Sodium vapor
(c) LED
(d) Deuterium
Click Here to join WhatsApp Group
71. Ostwald’s dilution law is applicable to
(a) Weak electrolytes
(b) Strong electrolytes
(c) Non-electrolytes
(d) All electrolytes
PHARMACOLOGY
72. Following statement is correct with respect to voltage sensitive calcium channels
(a) L-type Blocker: Nifedipine
(b) T-type Blocker: Verapamil
(c) N-type Blocker: Mibefradil
(d) R-type Blocker: Diltiazem
73. One of the following drugs is not meant for systemic use
(a) Netilmycin
(b) Sisomicin
(c) Neomycin
(d) Paromomycin
74. Which among the following is not used as NSAID
(a) Indomethacin extended release 75 mg
(b) Aspirin 75 mg
(c) Naproxen 500 mg
(d) Mefenamic acid 500 mg
75. Fight or Flight responses are mediated by
(a) Parasympathetic division of Autonomous nervous system
(b) Sympathetic division of Autonomous nervous system
(c) Serotonergic nervous system
(d) Histaminergic nervous system
76. Which one of the following is an autosomal dominant syndrome in its inheritance
(a) Gilbert’s syndrome
(b) Crigler-Najjar syndrome Type-I
(c) Dubin- Johnson syndrome
(d) Rotor syndrome
Click Here to Join Telegram Group
77. One of the following match is correct choose it
(a) M1 Acetylcholine receptors confined to brain M2 Acetylcholine receptors neural M3 Acetyl choline receptors are cardiac M4 Acetylcholine receptors glandular
(b) M1 Acetylcholine receptors neural M2 Acetylcholine receptors confined to brain M3 Acetylcho line receptors are cardiac M4 Acetylcholine receptors glandular
(c) M1 Acetylcholine receptors neural M2 Acetylcholine receptors cardiac M3 Acetylcholine receptors are confined to glandular M4 Acetylcholine receptors confined to brain
(d) M1 Acetylcholine receptors glandular M2 Acetylcholine receptors neural M3 Acetylcholine receptors are confined to brain M4 Acetylcholine receptors cardiac
78. ______ is a protein marker which can be detected within three hours of acute ischemic kidney injury from patient’s urine
(a) N-acetyl- β-D-glucose aminidase
(b) Glutathione-S-transferase
(c) Neutrophil gelatinase associated lipocalin
(d) γ-glutamyl transpeptidase
79. Which of the following genes responsible for graft rejection in humans
(a) Highly polymorphic HLA genes
(b) APP genes
(c) hMSH2 gene
(d) FMR1 gene
80. Match List I with List II
| LIST – I | LIST – II |
| 1. Vibrations in skeletal muscles of larynx | [P] Facial contraction |
| 2. Involuntary contraction of skeletal muscles that is regulated by the brain | [Q] Regulate voice |
| 3. Contraction of skeletal muscles in the legs | [R] Shivering |
| 4. Pull of skeletal muscles on attachments to skin of face | [S] Assists return of blood to the heart |
| [T] Causes facial expressions |
Choose the correct answer from the options given below
(a) 1-[Q], 2-[R], 3-[S], 4-[P]
(b) 1-[R], 2-[S], 3-[P], 4-[T]
(c) 1-[Q], 2-[R], 3-[S], 4-[T]
(d) 1-[R], 2-[S], 3-[T], 4-[P]
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
81. Most common type of megaloblastic anemia caused by malabsorption of vitamin B12 and characterized by decreased production of hydrochloric acid in the stomach and a deficiency of intrinsic factor is
(a) Iron deficiency anemia
(b) Sideroblastic anemia
(c) Pernicious anemia
(d) Aplastic anemia
82. Which of the following is not an ACE inhibitor
(a) Captopril
(b) Trandolapril
(c) Verapamil
(d) Lisinopril
83. Which of the following combination is correct
(a) Nucleoside reverse transcriptase inhibitors (NRTIs) – Ritonavir
(b) Protease inhibitors – Indinavir
(c) lntegrase inhibitors – Saquinavir
(d) Non-nucleoside reverse transcriptase inhibitors (NNRTIs) – Tenofovir
84. Choose the most appropriate answer
[P] Enterobius vermicularis-Pin worm [Q] Strongyloides stercoralis-Thread worm
[R] Wuchereria bancrofti-Filarial worm [S] Dracunculus medinensis-Guinea worm
(a) Only Q and R are correct
(b) Only P and Q are correct
(c) P, Q, R and S are correct
(d) Only Q, R and S are correct
85. activates G-protein gated potassium channel resulting in membrane hyperpolarization
(a) α1 adrenergic receptor
(b) α2 adrenergic receptor
(c) β1 adrenergic receptor
(d) β2 adrenergic receptor
86. Which among the following statement is correct with respect to their mechanism of antidiabetic action
(a) Dapagliflozin/Canagliflozin-Sodium glucose cotranspoti-2 inhibitors
(b) Glipizide/Gliclazide – Dipeptidyl peptidase-4 inhibitors
(c) Linagliptin/Alogliptin -AMPK activators
(d) Acarbose / Voglibose-K+ATP channel blockers
Click Here to join WhatsApp Group
87. Prominent biochemical features of Grave’s disease are
(a) Decreased ionized calcium in body fluids
(b) Decreased T4 and T3 in circulation
(c) Increased ionized calcium in body fluids
(d) Increased T4 and T3 in circulation
88. Laboratory findings of which one of the following disease include markedly elevated serum amylase levels during the first 24 hours followed by rising serum lipase levels within 72-96 hours
(a) Acute pancreatitis
(b) Cirrhosis of liver
(c) Jaundice
(d) Cystic fibrosis of lungs
89. Exenatide is a
(a) Glucagon like peptide 1 (GLP 1) receptor agonist
(b) Diphenyl Peptidase-4 (DPP4) inhibitors
(c) Facilitator of glucose transport across the cell
(d) Inhibitor of glucose absorption in the GIT
Download PHARMACY INDIA MOBILE APP from Google Play Store
90. Which of the following is a third generation Cephalosporin
(a) Cefazolin
(b) Cefuroxime
(c) Cefotaxime
(d) Cefepime
91. Deficiency of _____ enzyme is found in Hers’ disease
(a) Muscle glycogen phosphorylase
(b) Liver glycogen phosphorylase
(c) Phosphofructokinase
(d) Glucose-6-phosphatase
92. One of the following is correct match or mechanisms. Select it
(a) Methotrexate-Inhibition of microtubules, Vinca alkaloids -Inhibition of Purine synthesis, Bleomycin- Inhibition of RNA, 5-Fluoro Uracil-DNA damage
(b) Methotrexate-Inhibition of Purine synthesis, Vinca alkaloids -Inhibition of Micro tubules, Bleomycin-DNA damage, 5-Fluoro Uracil-inhibition of 2-deoxythymidylate
(c) Methotrexate-DNA damage, Vinca alkaloids-Inhibition of micro tubules, Bleomycin-Inhi bition of 2-deoxythymidylate. 5-Fluoro Uracil-RNA damage
(d) Methotrexate-DNA damage, Vinca alkaloids-RNA damage, Bleomycin-Inhibition of Purine synthesis, 5-Fluoro Uracil-Inhibition of 2-deoxythymidylate
93. The β- adrenergic antagonist propranolol (20-120 mg/kg) is prescribed to around 50% of maniac patients receiving Lithium is to mitigate the side effect
(a) Anxiety
(b) Tremor
(c) Hypertension
(d) Bradycardia
94. Which class of antibody opsonizes antigens for phagocytosis through two different path ways
(a) Immunoglobulin G (IgG)
(b) Immunoglobulin M (IgM)
(c) Immunoglobulin A (IgA)
(d) Immunoglobulin E (IgE)
95. Anti-rheumatoid drug which is contraindicated in patients with renal and hepatic impairment
(a) Sulfasalazine
(b) Methotrexate
(c) Corticosteroids
(d) Azathioprine
96. Which among the following is an amino-alcohol analogue that has weak visceral anticholinergic activity but is a strong nicotinolytic drug
(a) Biperiden
(b) Orphenadrine
(c) Poldine
(d) Propantheline
97. Which one of the following interferes with the release of cholinergic neurotransmitter, acetylcholine by the neurons of autonomic nervous system
(a) Reserpine
(b) Guanethidine
(c) Hemicholinium
(d) Botulinum toxin
98. Which one of the following types of hepatitis can lead to fulminant hepatitis causing massive hepatic cell death more frequently among infected pregnant women, showing third trimester mortality as high as 30%
(a) Hepatitis A
(b) Hepatitis B
(c) Hepatitis C
(d) Hepatitis E
99. Phocomelia is caused by
(a) Glibenclamide
(b) Indapamide
(c) Xipamide
(d) Thalidomide
100. Valproate and Carbamazepine can be used as first line drugs for the management of following type of seizure
(a) Both partial seizure and Tonic-clonic seizure
(b) Both Tonic-clonic seizure and Status epilepticus
(c) Only Febrile seizures
(d) Both Febrile seizures and Status epilepticus
Follow us on Instagram
101. Which of the following is not a Sulphonamide derivative
(a) Almotriptan
(b) Sumatriptan
(c) Rizatriptan
(d) Naratriptan
102. Which phase of cell cycle is the shortest phase in terms of time
(a) G1 (b) S (c) M (d) G2
PHARMACOGNOSY
103. The following are adulterants of clove EXCEPT one, choose the most appropriate option
(a) Mother Clove
(b) Clove Stalk
(c) Blown Clove
(d) Clove Bud
104. Unicellular conical, warty trichomes, paracytic stomata, xylem vessels with annular thick ening are important microscopical features of which plant
(a) Datura metel
(b) Cassia angustifolia
(c) Digitalis purpurea
(d) Atropa belladonna
105. Sesquiterpenes are biosynthesized from in plants
(a) Farnesyl-pyrophosphate
(b) Geranyl farnesyl pyrophosphate
(c) Terpenes
(d) Degraded products of terpenes
106. The alcohol solution of Sudan-III and tincture of alkana are the reagents used for identification of following type of secondary metabolites
(a) Resins
(b) Alkaloids
(c) Fixed oils
(d) Volatile oils
107. Aloe contains _______ type of glycosides
(a) C-glycosides
(b) O-glycosides
(c) S-glycosides
(d) N-glycosides
108. Ayurvedic fermented preparation includes
(a) Churnas
(b) Tailas
(c) Bhasmas
(d) Aristas and Asavas
Click Here to Join Telegram Group
109. Modified borntrager’s test is used to detect the presence of which type of glycosides
(a) O-type of glycosides
(b) C-type of glycosides
(c) S-type of glycosides
(d) N-type of glycosides
110. Terpene indole alkaloid derived from L-Tryptophan via Secologanin is
(a) Morphine
(b) Codeine
(c) Ajamalicine
(d) Thebaine
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
111. Chemically volatile oils differ from fixed oils in one of the following characters
(a) Mixtures of eleoptenes and steroptenes
(b) Presence of flavonoids
(c) Presence of plant acids
(d) Hydrophilic in nature
112. Which one of the following drug is skeletal muscle relaxant
(a) Datura stramonium
(b) Atropa belladonna
(c) Hyoscyamus niger
(d) Chondrodendron tomentosum
113. Match the photo pharmaceutical with the plant species from which they are produced
| LIST – I (COMPOUND) | LIST – II (PLANT SPECIES) |
| 1. Cardenolides | [P] Ruta graveolens |
| 2. Rutin | [Q] Catharanthus roseus |
| 3. Ajamalicine | [R] Papaver simnifera |
| 4. Codeine | [S] Digitalis lanata |
Choose the correct answer from the options given below
(a) 1-[S], 2-[P], 3-[Q], 4-[R]
(b) 1-[P], 2-[R], 3-[Q], 4-[S]
(c) 1-[R], 2-[P], 3-[Q], 4-[S]
(d) 1-[Q], 2-[S], 3-[P], 4-[R]
OTHER SUBJECTS
114. Carbohydrates have hydrogen oxygen atom ratio of
(a) 1 : 2 (b) 3 : 1 (c) 1 : 3 (d) 2: 1
115. Autoimmunity refers to
(a) An automatic trigger of the immune system directed against a specific pathogen
(b) Failure to distinguish between self and non-self
(c) An automatic segregation of T and B cells
(d) Failure of B-cells to interact with T-cells
Click Here to join WhatsApp Group
116. Which of the following is acellular
(a) Bacteria
(b) Fungus
(c) Virus
(d) Amoeba
117. Recombination process in a cell occurring through the mediation of phages is called
(a) Transfection
(b) Transduction
(c) Conjugation
(d) Transformation
118. The Schick test is used to determine susceptibility to
(a) Measles
(b) Diphtheria
(c) Polio
(d) Typhoid
119. Absorption of Vitamin B12 is facilitated by
(a) Hydrogel
(b) Glycoprotein
(c) Lipoprotein
(d) Mucoprotein
120. Enzyme asparaginase is obtained from
(a) Clostridium histolyticum
(b) Bacillus subtilis
(c) Erwinia carotovora
(d) Kluyveromyces lactis
Download PHARMACY INDIA MOBILE APP from Google Play Store
121. In 1798 Edward Jenner published his work on
(a) Vaccination
(b) Prescription writing
(c) Isolation of morphine
(d) Isolation of codeine
122. Modified Lowry’s procedure is used to characterize
(a) Protein-Content in allergen product
(b) Protein profile in allergen product
(c) Potency of allergen product
(d) Storage condition of allergen product
123. Match List I with List II
| LIST – I | LIST – II |
| 1. Penicillin | [P] Single cell protein |
| 2. Pruteen | [Q] Ion exchange chromatography for recovery |
| 3. Streptomycin | [R] Primary metabolite |
| 4. Amino acids | [S] Phenyl acetic as precursor |
Choose the correct answer from the options given below
(a) 1-[Q], 2-[P], 3-[S], 4-[R]
(b) 1-[S], 2-[P], 3-[Q], 4-[R]
(c) 1-[R], 2-[Q], 3-[P], 4-[S]
(d) 1-[Q], 2-[R], 3-[S], 4-[P]
124. Who is the primary source of information for doctors prescribing behaviour
(a) Competitor
(b) Wholesaler
(c) Fellow doctor
(d) Retailer
125. The good management principle revolves around the three R’s these are
(a) Ration, Rotation and Responsibility
(b) Reward, Recognition and Responsibility
(c) Research, Recreation and Responsibility
(d) Reverse engineering, Research and Responsibility
ANSWERS
|
1-c |
2-a |
3-b |
4-c |
5-c |
6-d |
7-b |
8-a |
9-a |
10-b |
|
11-a |
12-a |
13-d |
14-b |
15-b |
16-b |
17-d |
18-c |
19-c |
20-b |
|
21-a |
22-a |
23-a |
24-b |
25-d |
26-d |
27-d |
28-c |
29-d |
30-d |
|
31-b |
32-d |
33-d |
34-a/c |
35-c |
36-a |
37-b |
38-a |
39-* |
40-* |
|
41-a |
42-d |
43-c |
44-d |
45-a |
46-b |
47-c |
48-a |
49-b |
50-b |
|
51-b |
52-b |
53-a |
54-a |
55-c |
56-a |
57-b |
58-b |
59-a |
60-d |
|
61-b |
62-d |
63-c |
64-a |
65-a |
66-a |
67-a |
68-a |
69-d |
70-d |
|
71-a |
72-a |
73-c |
74-b |
75-b |
76-a |
77-c |
78-c |
79-a |
80-c |
|
81-c |
82-c |
83-b |
84-c |
85-b |
86-a |
87-d |
88-a |
89-a |
90-c |
|
91-b |
92-b |
93-b |
94-a |
95-b |
96-a |
97-d |
98-a |
99-d |
100-a |
|
101-c |
102-c |
103-d |
104-b |
105-a |
106-d |
107-a |
108-d |
109-b |
110-c |
|
111-a |
112-d |
113-a |
114-d |
115-b |
116-c |
117-c |
118-b |
119-b |
120-c |
|
121-a |
122-a |
123-b |
124-d |
125-b |
|
||||
<<<<<<<<<<<<JOIN US>>>>>>>>>>>>>>>>
| Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates | Click Here |
| Follow us on Instagram | Click Here |
| Download PHARMACY INDIA MOBILE APP from Google Play Store | Click Here |
| Follow us on LinkedIn | Click Here |



