Methotrexate IUPAC Nomenclature, SAR, Physicochemiscal properties, Synthesis, Mechanism of Action and Uses. Unravel the intricacies of its IUPAC nomenclature and immerse yourself in the depths of its physicochemical properties. Grasp the essence of Methotrexate’s activity through its structural components. Marvel at its dihydrofolate moiety, crucial for interaction with dihydrofolate reductase (DHFR) and enzyme inhibition. Uncover the synthetic pathways that lead to Methotrexate’s creation. Explore its mechanism of action, where it inhibits dihydrofolate reductase (DHFRase), disrupting de novo purine synthesis and amino acid interconversion crucial for cellular प्रोसेसेज।
In the late 1940s, aminopterin, a compound closely related to methotrexate, was discovered. Aminopterin was the first known folic acid antagonist and was initially used in the treatment of leukemia. In the early 1950s, researchers at Lederle Laboratories, led by Dr. Sidney Farber, introduced methotrexate as a treatment for childhood leukemia. Methotrexate proved to be effective in inducing remission in pediatric leukemia patients. Methotrexate continued to be researched and applied in various medical conditions. Its use extended to autoimmune diseases such as psoriasis and inflammatory bowel diseases like Crohn’s disease.
IUPAC nomenclature
(2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid
Chemical Structure

Physicochemical Properties
1. Molecular Formula: C20H22N8O5
2. Molecular Weight: 454.44 g/mol
3. Solubility: Methotrexate is sparingly soluble in water but dissolves well in dilute solutions of alkali hydroxides and sparingly soluble in alcohol.
4. Melting Point: Methotrexate does not have a specific melting point. It decomposes before reaching a boiling point.
5. Appearance: Methotrexate is commonly found as a yellow to orange, crystalline powder or as small yellow-orange crystals.
6. Acidity (pKa): The pKa values of methotrexate are approximately 0.8, 1.7, and 3.75.
7. Chemical Stability: Methotrexate should be stored in a cool, dry place and protected from light. It is chemically stable under recommended storage conditions.
SAR of Methotrexate
1. Dihydrofolate Moiety: Methotrexate contains a dihydrofolate moiety, which is essential for its interaction with DHFR. The dihydrofolate structure is crucial for binding to the active site of the enzyme and inhibiting its activity. Modifications to this moiety can significantly impact the drug’s effectiveness.
2. Pteridine Ring: Methotrexate features a pteridine ring, which is structurally similar to the pteridine ring found in naturally occurring folates. This resemblance allows methotrexate to competitively inhibit DHFR, disrupting the folate metabolism pathway in cells.
3. Glutamate Side Chain: Methotrexate has a glutamate side chain. The length and structure of this side chain are important for the drug’s activity. Changes in the glutamate side chain can affect the drug’s affinity for the enzyme and its overall potency.
4. Polyglutamation: Methotrexate can be polyglutamated within cells, which involves the addition of multiple glutamate residues to the drug molecule. Polyglutamation enhances the drug’s retention within cells and prolongs its inhibitory effects on DHFR.
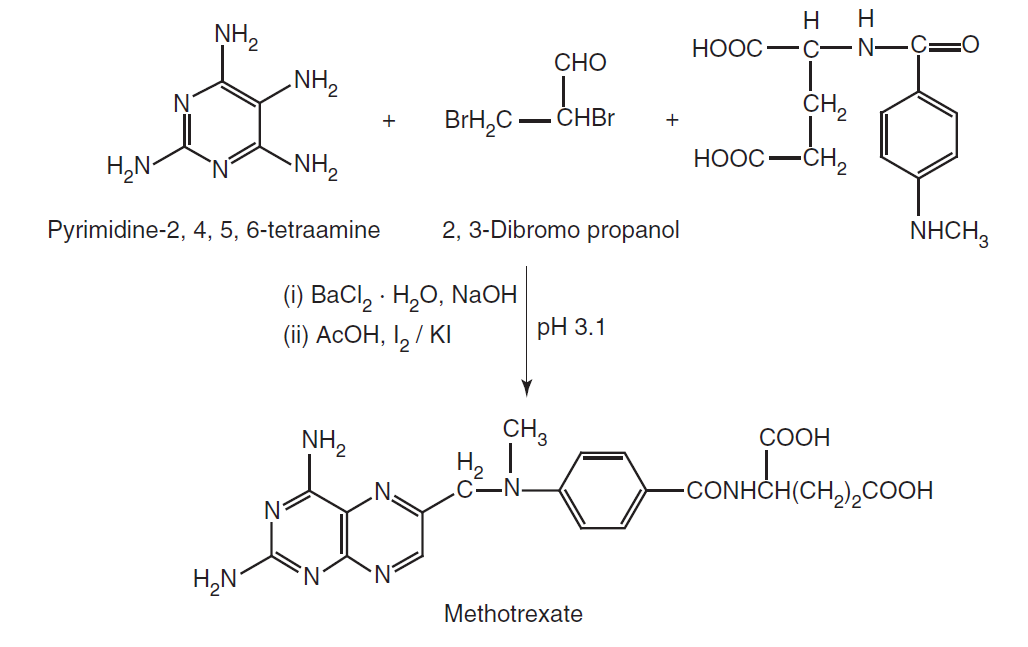
Synthesis
Mechanism of action
These drugs inhibit dihydrofolate reductase (DHFRase), which converts the dihydrofolic acid to tetrahydro folicacid, the co-enzyme required for one carbon transfer reaction in de novo purine synthesis and amino acid interconversion.
Uses
1. Acute lymphocytic leukaemia
2. Acute lymphoblastic leukaemia
3. Breast cancer
4. pidermoid cancer of the head, neck, and lung cancer
Acetazolamide: SAR, Synthesis, Mechanism, and Uses
Promethazine HCl SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses
Cimetidine SAR, Physicochemical Properties, IUPAC Nomenclature, Mechanism of action and Uses
Download B. Pharma 1st Semester Model Papers
B. Pharma 4th Semester Model Papers
Subscribe our PHARMACY INDIA Youtube Channel for more Pharma Updates
Click here to follow us on Instagram
Click here to Join our Telegram Channel
Download PHARMACY INDIA MOBILE APP from Google Play Store